Kerala Blood Donors
Tuesday, July 12, 2011
Friday, June 10, 2011
Wednesday, November 10, 2010
Tuesday, November 9, 2010
National Blood Policy (India)
National Blood Policy (India)
INTRODUCTION:
A well organised Blood Transfusion Service (BTS) is a vital component of any health care
delivery system. An integrated strategy for Blood Safety is required for elimination of transfusion
transmitted infections and for provision of safe and adequate blood transfusion services to the
people. The main component of an integrated strategy include collection of blood only from
voluntary, non-remunerated blood donors, screening for all transfusion transmitted infections and
reduction of unnecessary transfusion.
The Blood Transfusion Service in the country is highly decentralised and lacks many vital
resources like manpower, adequate infrastructure and financial base. The main issue, which
plagues blood banking system in the country, is fragmented management. The standards vary from
State to State, cities to cities and centre to centre in the same city. In spite of hospital based
system, many large hospitals and nursing homes do not have their own blood banks and this has
led to proliferation of stand-alone private blood banks.
The blood component production/availability and utilisation is extremely limited. There is
shortage of trained health-care professionals in the field of transfusion medicine.
For quality, safety and efficacy of blood and blood products, well-equipped blood centres with
adequate infrastructure and trained manpower is an essential requirement. For effective clinical
use of blood, it is necessary to train clinical staff. To attain maximum safety, the requirements of
good manufacturing practices and implementation of quality system moving towards total quality
management, have posed a challenge to the organisation and management of blood transfusion
service.
Thus, a need for modification and change in the blood transfusion service has necessitated
formulation of a National Blood Policy and development of a National Blood Programme which
will also ensure implementation of the directives of Supreme Court of India - 1996.
MISSION STATEMENT:
The policy aims to ensure easily accessible and adequate supply of safe and quality blood and
blood components collected / procured from a voluntary non-remunerated regular blood donor in
well equipped premises, which is free from transfusion transmitted infections, and is stored and
transported under optimum conditions. Transfusion under supervision of trained personnel for all
who need it irrespective of their economic or social status through comprehensive, efficient and a
total quality management approach will be ensured under the policy.
11. OBJECTIVES OF THE POLICY:
To achieve the above aim, the following objectives are drawn:
1.To reiterate firmly the Govt. commitment to provide safe and adequate quantity of blood, blood
components and blood products.
2.To make available adequate resources to develop and re-organise the blood transfusion services
in the entire country.
3.To make latest technology available for operating the blood transfusion services and ensure its
functioning in an updated manner.
4. To launch extensive awareness programmes for donor information, education, motivation,
recruitment and retention in order to ensure adequate availability of safe blood.
5. To encourage appropriate clinical use of blood and blood products.
6. To strengthen the manpower through human resource development.
7. To encourage Research & Development in the field of Transfusion Medicine and related
technology.
8. To take adequate regulatory and legislative steps for monitoring and evaluation of blood
transfusion services and to take steps to eliminate profiteering in blood banks.
OBJECTIVE – 1:
To reiterate firmly the Govt. commitment to provide safe and adequate quantity of blood,
blood components and blood products.
STRATEGY:
1.1. A national blood transfusion Programme shall be developed to ensure establishment of
non-profit integrated National and State Blood Transfusion Services in the country.
1.1.1 National Blood Transfusion Council (NBTC) shall be the policy formulating apex body in
relation to all matters pertaining to operation of blood centres. National AIDS Control
Organisation (NACO) shall allocate a budget to NBTC for strengthening Blood Transfusion
Service.
1.1.2 State/UT Blood Transfusion Councils shall be responsible for implementation of the Blood
Programme at State/UT level, as per the recommendations of the National Blood Transfusion
Council.
1.1.3 Mechanisms for better co-ordination between NBTC and SBTCs shall be developed by the
NBTC.
1.1.4 Mechanisms shall be developed to monitor and periodically evaluate the implementation of
the National Blood Programme in the country.
1.1.5 The enforcement of the blood and blood products standards shall be the responsibility of
Drugs Controller General India ) as per Drugs and Cosmetics Act/Rules, with assistance from
identified experts.
1.1.6 NBTC shall ensure involvement of other Ministries and other health programmes for various
activities related to Blood transfusion services.
1.2.Trading in blood i.e. Sale & purchase of blood shall be prohibited.
1.2.1 The practice of replacement donors shall be gradually phased out in a time bound
programme to achieve 100% voluntary non-remunerated blood donation programme.
1.2.1.1 State/UT Blood Transfusion Councils shall develop an action plan to ensure phasing out of
replacement donors.
1.3 The following chain of Transfusion Services shall be promoted for making available of safe
blood to the people.
1.3.1 State Blood Transfusion Councils shall organise the blood transfusion service through the
network of Regional Blood Centres and Satellite Centres and other Government, Indian Red Cross
Society & NGO run blood centres and monitor their functioning. All Regional Centres shall be
assigned an area around in which the other blood banks and hospitals which are linked to the
regional centre will be assisted for any requirement and shall be audited by the Regional Centre. It
will also help the State Blood Transfusion Council in collecting the data from this region.
1.3.2 The Regional Centres shall be autonomous for their day to day functioning and shall be
guided by recommendations of the State/UT Blood Transfusion Councils. The Regional Centre
shall act as a referral centre for the region assigned to it.
1.3.3 NBTC shall develop the guidelines to define NGO run blood centres so as to avoid
profiteering in blood banking.
1.4 Due to the special requirement of Armed Forces in remote border areas, necessary
amendments shall be made in the Drugs & Cosmetics Act/Rules to provide special licences to
small garrison units. These units shall also be responsible for the civilian blood needs of the
region.
OBJECTIVE – 2:
To make available adequate resources to develop and re-organise the blood transfusion
service in the entire country.
STRATEGY:
2.1 National & State/UT Blood Transfusion Councils shall be supported/ strengthened financially
by pooling resources from various existing programmes and if possible by raising funds from
international / bilateral agencies.
2.2 Efforts shall be directed to make the blood transfusion service viable through non-profit
recovery system.
2.2.1. National Blood Transfusion Council shall provide guidelines for ensuring non-profit cost
recovery as well as subsidised system.
2.2.2. Efforts shall be made to raise funds for the blood transfusion service for making it
self-sufficient.
2.2.3. The mechanism shall be introduced in government sector to route the amounts received
through cost recovery of blood/blood components to the blood banks for improving their services.
OBJECTIVE – 3:
To make latest technology available for operating the blood transfusion services and ensure
its functioning in an updated manner.
STRATEGY:
3.1 Minimum standards for testing, processing and storage shall be set and ensured.
3.1.1. Standards, Drugs & Cosmetics Act/Rules and Indian Pharmacopoeia shall be updated as and
when necessary.
3.1.2. All mandatory tests as laid down under provisions of Drugs & Cosmetics Act/Rules shall be
enforced.
3.1.3. Inspectorate of Drugs Controller of India and State FDA shall be strengthened to ensure
effective monitoring.
3.1.4. A vigilance cell shall be created under Central/State Licensing Authorities.
3.2. A Quality System Scheme shall be introduced in all blood centres.
3.2.1. Quality Assurance Manager shall be designated at each Regional Blood Centre/any blood
centre collecting more than 15,000 units per year to ensure quality control of Blood & its
components in the region assigned. He shall be exclusively responsible for quality assurance only.
3.2.2 Every blood centre shall introduce an internal audit system to be followed by corrective
actions to reduce variations in Standard Operating Procedures(SOPs) as a part of continuous
improvement programme.
3.2.3. Regular workshops on the subject of quality assurance shall be conducted to update the
personnel working in blood centres.
3.2.4. Regular proficiency testing of personnel shall be introduced in all the blood centres.
3.3. An External Quality Assessment Scheme (EQAS) through the referral laboratories approved
by the National Blood Transfusion Council shall be introduced to assist participating centres in
achieving higher standards and uniformity.
3.3.1. Reference centres shall be identified in each State/UT for implementation of EQAS. All
blood centres shall be linked to these reference centres for EQAS.
3.3.2. NBTC shall identify a centre of national repute for quality control of indigenous as well as
imported consumables, reagents and plasma products.
3.4. Efforts shall be made towards indigenisation of kits, equipment and consumables used in
blood banks.
3.5. Use of automation shall be encouraged to manage higher workload with increased efficiency.
3.6. A mechanism for transfer of technology shall be developed to ensure the availability of
state-of-the-art technology from out side India .
3.7. Each blood centre shall develop its own Standard Operating Procedures on various aspects of
Blood Banking.
3.7.1. Generic Standard Operating Procedures shall be developed by the National Blood
Transfusion Council as guidelines for the blood centres.
3.8. All blood centres shall adhere to bio-safety guidelines as provided in the Ministry of Health &
Family Welfare manual "Hospital-acquired Infections : Guidelines for Control" and disposal of
bio-hazardous waste as per the provisions of the existing Biomedical Wastes(Management &
Handling) Rules - 1996 under the Environmental Protection Act - 1986.
OBJECTIVE – 4:
To launch extensive awareness programmes for blood banking services including donor
motivation, so as to ensure adequate availability of safe blood.
STRATEGY:
4.1 Efforts shall be directed towards recruitment and retention of voluntary, non-remunerated
blood donors through education and awareness programmes.
4.1.1 There shall not be any coercion in enrolling replacement blood donors.
4.1.2 The replacement donors shall be encouraged to become regular voluntary blood donors.
4.1.3 Activities of NGOs shall be encouraged to increase awareness about blood donation
amongst masses.
4.1.4.All blood banks shall have donor recruitment officer/donor organiser.
4.1.5. Each blood centre shall create and update a blood donor’s directory which shall be kept
confidential.
4.1.6. In order to increase the donor base specific IEC campaigns shall be launched to involve
youth in blood donation activities.
4.2. Enrolment of safe donors shall be ensured.
4.2.1Rigid adherence to donor screening guidelines shall be enforced.
4.2.2 At blood donation camps, appropriate attention shall be paid on donor enrolment and
screening in accordance with national standards instead of number of units collected.
4.2.3 A Counselor in each blood centre shall be appointed for pre and post donation counseling.
4.2.4 Result seeking donors shall be referred to a Blood Testing Centre (BTC) for post donation
information and counseling.
4.3 State/UT Blood Transfusion Councils shall recognise the services of regular voluntary
non-remunerated blood donors and donor organisers appropriately.
4.4 National/State/UT Blood Transfusion Councils shall develop and launch an IEC campaign
using all channels of communication including mass-media for promotion of voluntary blood
donation and generation of awareness regarding dangers of blood from paid donors and
procurement of blood from unauthorised blood banks/laboratories.
4.5 National / State / UT blood transfusion councils shall involve other departments / sectors for
promoting voluntary blood donations.
OBJECTIVE: 5:
To encourage appropriate clinical use of blood and blood products.
STRATEGY:
5.1 Blood shall be used only when necessary. Blood and blood products shall be transfused only to
treat conditions leading to significant morbidity and mortality that cannot be prevented or treated
effectively by other means.
5.2 National Guidelines on "Clinical use of Blood" shall be made available and updated as
required from time to time.
5.3 Effective and efficient clinical use of blood shall be promoted in accordance with guidelines.
5.3.1 State/UT Governments shall ensure that the Hospital Transfusion Committees are
established in all hospitals to guide, monitor and audit clinical use of blood.
5.3.2 Wherever appropriate, use of plasma expanders shall be promoted to minimise the use of
blood.
5.3.3 Alternative strategies to minimise the need for transfusion shall be promoted.
5.4 Education and training in effective clinical use of blood shall be organised.
5.4.1 Medical Council of India shall be requested to take following initiatives:
5.4.1.1 To introduce Transfusion Medicine as a subject at undergraduate and all post graduate
medical courses.
5.4.1.2 To introduce posting for at least 15 days in the department of transfusion medicine during
internship.
5.4.1.3 To include Transfusion Medicine as one of the subjects in calculating credit hours for the
renewal of medical registration by Medical Council of India, if it is introduced.
5.4.2 CME and workshops shall be organised by State Blood Transfusion Councils in
collaboration with professional bodies at regular intervals for all clinicians working in private as
well public sector in their States.
5.5 Blood and its components shall be prescribed only by a medical practitioner registered as per
the provisions of Medical Council Act - 1956.
5.6 Availability of blood components shall be ensured through the network of regional centres,
satellite centres and other blood centres by creating adequate number of blood component
separation units.
5.7 Appropriate steps shall be taken to increase the availability of plasma fractions as per the need
of the country through expanding the capacity of existing centre and establishing new centres in
the country.
5.8 Adequate facilities for transporting blood and blood products including proper cold-chain
maintenance shall be made available to ensure appropriate management of blood supply.
5.9 Guidelines for management of blood supply during natural and man made disasters shall be
made available.
OBJECTIVE: 6:
To strengthen the manpower through Human Resource Development.
STRATEGY:
6.1 Transfusion Medicine shall be treated as a speciality.
6.1.1 A separate Department of Transfusion Medicine shall be established in Medical Colleges.
6.1.2 Medical Colleges/Universities in all States shall be encouraged to start PG degree (MD in
transfusion medicine) and diploma courses in Transfusion Medicine.
6.1.3 PG courses for technical training in transfusion medicine (PhD / MSc) shall also be
encouraged.
6.2 In all the existing courses for nurses, technicians and pharmacists, Transfusion Medicine shall
be incorporated as one of the subjects.
6.3 In-service training programmes shall be organised for all categories of personnel working in
blood centres as well as drug inspectors and other officers from regulatory agencies.
6.4 Appropriate modules for training of Donor Organisers/Donor Recruitment Officers shall be
developed to facilitate regular and uniform training programmes to be conducted in all States
6.4.1 Persons appointed as Donor Organisers/Donor Recruitment Officers shall undergo training
for Donor Motivation and Recruitment organised by State Blood Transfusion Councils.
6.5 Short orientation training cum advocacy programmes on donor motivation and recruitment
shall be organised for Community Based Organisations(CBOs)/NGOs who wish to participate in
Voluntary Blood Donor Recruitment Programme.
6.6 Inter-country and intra-country exchange for training and experience of personnel associated
with blood centres shall be encouraged to improve quality of Blood Transfusion Service.
6.7 States/UTs shall create a separate cadre and opportunities for promotions for suitably trained
medical and para medical personnel working in blood transfusion services.
OBJECTIVE: 7:
To encourage Research & Development in the field of Transfusion Medicine and related
technology.
STRATEGY:
7.1 A corpus of funds shall be made available to NBTC/SBTCs to facilitate research in transfusion
medicine and technology related to blood banking.
7.2 A technical resource core group at national level shall be created to co-ordinate research and
development in the country. This group shall be responsible for recommending implementation
of new technologies and procedures in coordination with DC(I).
7.3 Multi-centric research initiatives on issues related to Blood Transfusion shall be encouraged.
7.4 To take appropriate decisions and/or introduction of policy initiatives on the basis of factual
information, operational research on various aspects such as various aspects of Transfusion
Transmissible Diseases, Knowledge, Attitude and Practices (KAP) among donors, clinical use of
blood, need assessment etc shall be promoted.
7.5 Computer based information and management systems shall be developed which can be used
by all the centres regularly to facilitate networking.
OBJECTIVE: 8:
To take adequate legislative and educational steps to eliminate profiteering in blood banks.
STRATEGY:
8.1 For grant/renewal of blood bank licenses including plan of a blood bank, a committee,
comprising of members from State/UT Blood Transfusion Councils including Transfusion
Medicine expert, Central & State/UT FDAs shall be constituted which will scrutinise all
applications as per the guidelines provided by Drugs Controller General India ).
8.2 Fresh licenses to stand-alone blood banks in private sector shall not be granted. Renewal of
such blood banks shall be subjected to thorough scrutiny and shall not be renewed in case of
non-compliance of any condition of licence.
8.3 All State/UT Blood Transfusion Councils shall develop a State Action Plan for the State/UT
Blood Transfusion Service where in Regional Blood Transfusion Centres shall be identified.
These centres shall be from Government, Indian Red Cross Society or other NGO run blood banks
of repute. Approved regional blood centres/government blood centres/Indian red cross blood
centres shall be permitted to supply blood and blood products to satellite centres which are
approved by the committee as described in para 8.1. The Regional Centre shall be responsible for
transportation, storage, cross-matching and distribution of blood and blood products through
satellite centres.
8.4 A separate blood bank cell shall be created under a senior officer not below the rank of
DDC(I) in the office of the DC(I) at the headquarter. State/UT Drugs Control Department shall
create such similar cells with the trained officers including inspectors for proper inspection and
enforcement.
8.5 As a deterrent to paid blood donors who operate in the disguise of replacement donors,
institutions who prescribe blood for transfusion shall be made responsible for procurement of
blood for their patients through their affiliation with licensed blood centres.
8.6 States/UTs shall enact rules for registration of nursing homes wherein provisions for affiliation
with a licensed blood bank for procurement of blood for their patients shall be incorporated.
8.7 The existing provisions of drugs & Cosmetics Rules will be periodically reviewed to introduce
stringent penalties for unauthorised/irregular practices in blood banking system.
Source: http://www.naco.nic.in 04/15/2003
Low rate of voluntary blood donation in Kerala, say experts
Low rate of voluntary blood donation in Kerala, say experts | |||
| |||
Friday, November 5, 2010
Voluntary Blood Donation FAQ
Voluntary Blood Donation FAQ
A: Blood is the red coloured fluid flowing continuously in our body's circulatory system. About 1/12th of the body weight of a healthy individual is blood. On an average there are about 5 - 6 litres of blood present.
A: Blood contains mainly a fluid called plasma in which are suspended cellular elements. Three types of cells - Red Blood Cells or RBC's, White Blood Cells or WBC's and tiny platelets form the cellular element.
A: (a) Plasma: acts as a vehicle to carry many substances like glucose, fats, and proteins, enzymes, and hormones etc., in addition to the blood cells.
(b) Red Cells: carry oxygen from lungs to various body tissues and take back carbon dioxide from the cells and tissues to be thrown out of body in the form of exhaled air.
(c) White cells: mainly act as body scavengers and guards. They help in the immune system of the body and act as defence forces of the body killing the bacteria or any other organisms entering the body.
(d) Platelets: help in the clotting and coagulation of blood. We have experienced in our life that whenever we get injured the bleeding stops after a few minutes. This is brought about by a mechanism called clotting of blood in which platelets plays a very vital role.
A: Blood consists of RBCs, WBCs, platelets suspended in plasma. In early embryonic life blood cells are formed in liver and spleen. But by the fifth month the Haemopoiesis (i.e., formation of blood.) occurs in bone marrow and lymphatic tissues. At birth the entire bone marrow is red and active. Gradually as the child grows, the marrow remains red only in the flat bones and vertebrae. The RBC, granulocytes of WBC and platelets are produced mainly by bone marrow. The lymphocytes, monocytes, plasma cells are formed in the lymphoid and Reticulo Endothelial tissues. The orderly proliferation of the cells in the bone marrow and their release into circulation is carefully regulated according to the needs of body. Every day, new blood cells are being produced in the bone marrow and every day old cells are dying and being removed from the body.
Red blood cells have a life of 120 days and when it becomes old and senile it is thrown out. White cells live for a few days and platelets for a few hours. Thus daily new cells are added to the circulation and old are removed from it.
A: Haemoglobin is a substance present in the red cells. It is helpful in carrying oxygen and carbon dioxide. On an average, in a healthy male it should be between 14 - 16 gm % and in a female it should be about 12 - 14 gm %. This is also being daily synthesized and the new is replacing the old stock.
A: Every individual has two types of blood groups. The first is called the ABO - grouping and the second type is called Rh - grouping.
In the ABO - group there are four categories namely A Group, B Group, O Group and AB Group.
In the Rh - Group either the individual is Rh-positive, or Rh-negative. Rh is a factor called as Rhesus factor that has come to us from Rhesus monkeys.
Thus each and very human being will fall in one of the following groups.
A positive or A negative
B positive or B negative
O positive or O negative
AB positive or AB negative
There are also some sub groups as well as a few other classifications.
A positive or A negative
B positive or B negative
O positive or O negative
AB positive or AB negative
There are also some sub groups as well as a few other classifications.
A: For all practical and routine purposes, it is ideal to transfuse to the patient the same group of blood which he belongs to. It is only under very dire emergency that we take O group as universal donor and AB groups as universal recipient. Under no circumstances O group can get any other blood except O. Similarly A group patient cannot be given B group blood and vice versa.
A: This is due to the reason that, the blood of A Group people contains anti - B antibodies. In B group people there are anti - An antibodies. If we give A group blood to a B group patient, it is bound to be incompatible and will result in serious consequences.
A: A patient with Rh-negative blood cannot be given Rh-positive blood as the antigen-antibody REACTIONS WILL RESULT IN SEVERE consequences.
In cases where a woman has Rh negative and her husband has Rh positive, the first child with Rh positive may be normal. But subsequently the woman may not conceive or may have repeated abortions. There may be intra uterine fetal death. If the child born is alive, it will suffer from a fatal disease called "Erythroblastosis Foetalis
A: Blood is collected in plastic bags which contain a watery fluid which prevents blood from getting coagulated. On an average we draw about 450 ml. of blood from a person, depending on the weight of the donor. This blood, plus the amount of anti coagulant present in the bottle or bag, is known as one unit of blood.
A: Scientists have tried a lot but so far they are not successful. Only the blood of a human being can be transfused to a human patient.
A: Whole blood can be stored up to 35 days, when kept in CPDA anti coagulant solution and refrigerated at 2 - 4 deg C. But the demand is so great that blood hardly ever remains in storage for so long and is used much before expiry.
A: Yes! Now with technical advancements, we can make components of blood and store them. For example, plasma can be separated from whole blood and stored up to one year in frozen state at -80 deg C temperature or below. This is called Fresh Frozen Plasma. Similarly there are other components like Platelet Rich Plasma; Platelet Concentrate (can be stored as a life saving measure upto 5 days now at 22- 24 degrees C in a platelet incubator and agitator); Cryoprecipitate (which is very useful in treating bleeding disorders due to the deficiency of factor VIII and IX); Factor VIII and IX; Albumin, Globulin and many others.
In most progressive blood banks more than 85 % of the blood collected is converted into components and stored. This is because many patients do not require whole blood. For example, a patient whose hemoglobin is low and is therefore anemic may just require Packed Cells i.e. only red cells; a patient with burns may need more of plasma than cells; a patient with hemophilia may require only Factor VIII.
Now with the advent of Cell-separators we can directly draw a particular component from the donor, while rests of the blood constituents go back to the donor.
Transfusion
A: There are many situations in which patients need blood to stay alive:
- A patient needs blood after a major accident in which there is loss of blood.
- No major surgery is performed without blood as there is bound to be blood loss.
- On an average, for every open heart surgery about 6 units of blood is required.
- In miscarriage or childbirth, cases the patient may need large amount of blood to be transfused for saving her life and also the child's.
- For patients with blood diseases like severe Anemia especially Aplastic Anaemias, Leukemia’s (blood cancer), Haemophilia (bleeding disorder), and Thalassaemia etc. repeated blood transfusions are the only solution.
- In many other situations like poisoning, drug reactions, shock, burns, blood transfusion is the only way to save precious human life.
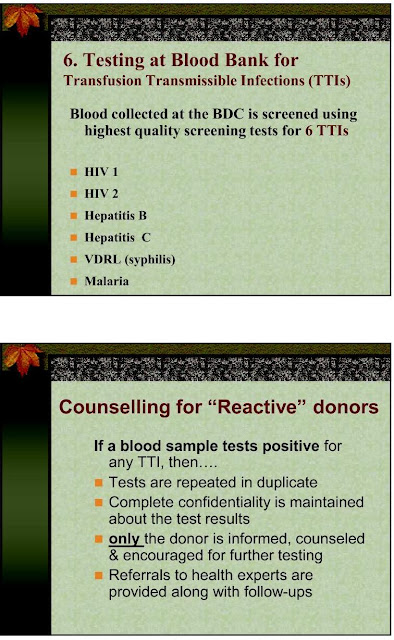
A: Yes. ALL the blood in our blood bank is tested for AIDS, VDRL, jaundice (HBsAg, HCV), malaria etc. using the latest technology.
Q: What happens to patients in transfusions with incompatible blood (mismatched blood)? A: The following symptoms may occur after only a few ml. of blood have been given:
1. Patient complains of shivering, restlessness, nausea, and vomiting. There is pericardial and lumbar pain.
2. Cold, clammy skin with cyanosis.
3. Pulse rate increases, respiratory rate increases. Temperature increases to 38 to 40 deg C. [101 to 105 F].
4. Blood pressure falls and patient passes into a state of shock.
5. Haemoglobinaemia, haemoglobinurea (urine turns red); oliguria (urine becomes scanty or the urinary output is reduced) and anuria (total output of urine becomes 200 ml. a day)
6. Jaundice appears after a few hours and in some cases anuria persists and uremia develops. This may lead to death.
Donating Blood
A: There are three types of blood donors: -
(1) PROFESSIONAL DONORS - They sell their blood, which is of very poor quality and can transmit very dangerous diseases to the recipient. It is illegal to take blood from any professional donor.
(2) REPLACEMENT DONATION - Healthy relatives and friends of the patient give their blood, of any group, to the blood bank. In exchange, the required number of units in the required blood group is given.
(3) VOLUNTARY DONATION- Here a donor donates blood voluntarily. The blood can be used for any patient even without divulging the identity of the donor. This is the best type of blood donation where a motivated human being gives blood in an act of selfless service.
A: Any person within the age group of 18 - 60 years with a body weight as minimum 45 kgs, and having hemoglobin content as minimum 12.5 gm%.
A: The donor should eat at regular mealtimes and drink plenty of fluids.
A: The procedure is done by skilled, specially trained technicians and takes three to eight minutes. However, from start to finish (filling form, post donation rest etc) the entire process should take upwards of 35 minutes.
A: There may be a little sting when the needle is inserted, but there should be no pain during the donation.
A: Absolutely not, rather a donor after having given blood voluntarily gets a feeling of great pleasure, peace and bliss. Soon, within a period of 24 - 48 hours, the same amount of new blood gets formed in the body, which helps the donor in many ways. His own body resistance improves, the circulation improves, and he himself feels healthier than before.
A: Yes. The donor needs rest, preferably lying down, so that the amount of blood that has been donated soon gets poured into the circulation from the body pools in a natural way. The donor should take it easy for about 15 - 20 minutes.
A: Of course! Routine work is absolutely fine after the initial rest. Rigorous physical work should be avoided for a few hours.
A: After resting for a while a donor is given some liquid (fluid) to take. It may be a cup of coffee or milk or fruit juice along with a few biscuits or fruit. The donor needs no other special diet. A routine balanced diet is adequate. The donor's blood gets replenished within 24 - 48 hours.
A: The body replaces blood volume or plasma within 24 hours. Red cells need about four to eight weeks for complete replacement.
A: Three months time between donations is a very safe interval.
A: Yes, if the donor has suffered from any of the under-mentioned diseases: -
Fever: He should not have suffered from fever for the past 15 days.
Jaundice: A donor should not have his blood tested positive for AUSTRALIA ANTIGEN.
Blood transmitted diseases: Like Syphilis, Malaria, Filarial etc. debar a donor from donating blood till he is treated and is free from them.
Drugs: If a donor is taking drugs like Aspirin, anti-hypertensive, anti-diabetics, hormones, corticosteroids etc., he is unfit to donate blood.
AIDS. No person having HIV positive can be allowed to donate blood.
A: Yes, blood donation is a noble, selfless service! It gives the donor a feeling of joy and contentment. Also this is an expression of love for Mankind, as blood knows no caste, colour, creed, religion or race, country, continent or sex.
“Nobody can do everything, but everyone can do something.”
DONATE BLOOD SAVE LIVES………….
Subscribe to:
Posts (Atom)